- Personal Protection Face Mask PPE Gloves COVID 19 Test Kits Goggles Other
- Surgical Surgery Basic Surgical Instruments Microsurgical Instruments Neurosurgical Instruments Beauty
- Infusion Care Disposable Syringes Split bag Infusion Sets Disposable Blood Transfusion Catheters Medical Tapes and Adhesives Medical Disinfection
- Dental Consumables Cleaning Tools Auxiliary Tools Measuring Tools Orthodontic Materials Whitening Materials
- Orthopedic Consumables Prosthesis Series Fusion Device Series Bone Fixation Series
- Ophthalmology Consumables Auxiliary Class for Ophthalmology Cleaning Class for Ophthalmology Simple Instruments for Ophthalmology Measuring Instruments for Ophthalmology
- Home Health Care Medical Rehabilitation Health Massage Inspection
- Hospital Hardware Equipment Basic Equipment Refrigeration Equipment Pharmaceutical Machinery Equipment Medical Video Photography Equipment
- Medical Equipment and Production Equipment Physical Diagnostic and Treatment Equipment Imaging Instruments Analytical Instruments Disinfection Equipment Pet Medical
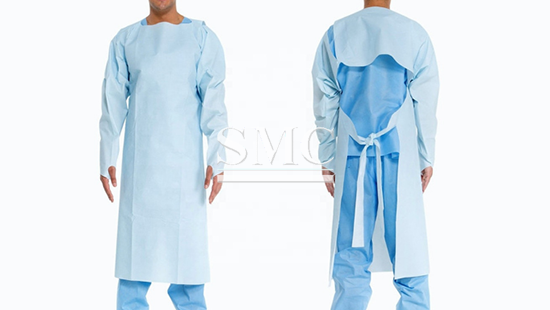
Disposable Isolation Gown with FDA CE
Gowns are examples of personal protective equipment used in health care settings. They are used to protect the wearer from the spread of infection or illness if the wearer comes in contact with potentially infectious liquid and solid material. They may also be used to help prevent the gown wearer from transferring microorganisms that could harm vulnerable patients, such as those with weakened immune systems. Gowns are one part of an overall infection-control strategy.
A few of the many terms that have been used to refer to gowns intended for use in health care settings, include surgical gowns, isolation gowns, surgical isolation gowns, nonsurgical gowns, procedural gowns, and operating room gowns, etc.
In 2004, the FDA recognized the consensus standard American National Standards Institute/Association of the Advancement of Medical Instrumentation (ANSI/AAMI) PB70:2003, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.” New terminology in the standard describes the barrier protection levels of gowns and other protective apparel intended for use in health care facilities and specifies test methods and performance results necessary to verify and validate that the gown provides the newly defined levels of protection:
· Level 1: Minimal risk, to be used, for example, during basic care, standard isolation, cover gown for visitors, or in a standard medical unit
· Level 2: Low risk, to be used, for example, during blood draw, suturing, in the Intensive Care Unit (ICU), or a pathology lab
· Level 3: Moderate risk, to be used, for example, during arterial blood draw, inserting an Intravenous (IV) line, in the Emergency Room, or for trauma cases
· Level 4: High risk, to be used, for example, during long, fluid intense procedures, surgery, when pathogen resistance is needed or infectious diseases are suspected (non-airborne)
Regardless of how the product is named (that is, isolation gown, procedure gown, or cover gown), when choosing gowns, look for product labeling that describes an intended use with the desired level of protection based on the above risk levels. Product names are not standardized.
Labeling that shows a product has been tested to and meets appropriate performance standards is one way for users and procurers to determine when to use a particular gown.
The performance of gowns is tested using consensus standards:
American Society for Testing and Materials (ASTM) F2407 is an umbrella document which describes testing for surgical gowns: tear resistance, seam strength, lint generation, evaporative resistance, and water vapor transmission.
Below is a summary of ASTM F2407 standard recognized by the FDA.
· Tensile Strength: ASTM D5034, ASTM D1682
· Tear resistance: ASTM D5587(woven), ASTM D5587 (nonwoven), ASTM D1424
· Seam Strength: ASTM D751 (stretch woven or knit)
· Lint Generation (ISO 9073 Part 10)
· Water vapor transmission (breathability) ASTM F1868 Part B, ASTM D6701 (nonwoven), ASTM D737-75
American National Standards Institute (ANSI) and the Association of the Advancement of Medical Instrumentation (AAMI): ANSI/AAMI PB70:2003 describes liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.



Disposable Gown Data Specification (cm)
Packaging of Disposable Isolation Gown:



here
for
price